hidden title
Biosafety Regulatory Support Services
Activities undertaken
- Actively engaged in biosafety issues related to Genetically Modified Organisms (GMOs) and new gene technologies
- Working closely with the nodal ministries and other concerned agencies
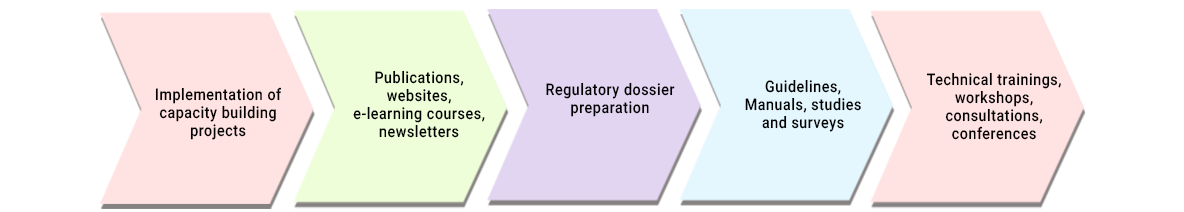
- Capacity building activities for multiple stakeholders through organizing conferences, workshops, technical trainings, consultations, publications, e-learning courses.
- Assistance in preparation of guidelines, manuals and resource documents
- Assisting organizations in regulatory data generation and compliance
- Expertise in preparation of well researched publications in multiple languages, newsletters, e-learning courses and websites for creating awareness
- Implementation of capacity building projects.
Key projects
- In-country project partner for the USAID supported South Asia Biosafety Programme (SABP) implemented by ILSI Research Foundation, USA, since 2005. Co-organize annual South Asia Biosafety Conferences since 2013 (seven so far in India, Bangladesh and Sri Lanka)
- Activities undertaken for more than ten GEF supported projects through international agencies like UNEP, FAO, UNDP and World Bank.
- Associated with Biosafety Capacity Building programmes in other countries such as Bhutan, Sri Lanka, Bangladesh and Malaysia.
- Played a key role in developing guidelines, manuals, resource documents in India, Sri Lanka and Bhutan.
- Prepared the proposed plan for establishment of Biotechnology Regulatory Authority of India (BRAI).
- Associated with preparation of national reports to relevant international agreements i.e. Convention on Biological Diversity (CBD) and the Cartagena Protocol on Biosafety (CPB).
- In-country project partner for the USAID supported South Asia Biosafety Programme (SABP) implemented by ILSI Research Foundation, USA, since 2005. Co-organize annual South Asia Biosafety Conferences since 2013 (seven so far in India, Bangladesh and Sri Lanka)
- Activities undertaken for more than ten GEF supported projects through international agencies like UNEP, FAO, UNDP and World Bank.
- Associated with Biosafety Capacity Building programmes in other countries such as Bhutan, Sri Lanka, Bangladesh and Malaysia.
- Played a key role in developing guidelines, manuals, resource documents in India, Sri Lanka and Bhutan.
- Prepared the proposed plan for establishment of Biotechnology Regulatory Authority of India (BRAI).
- Associated with preparation of national reports to relevant international agreements i.e. Convention on Biological Diversity (CBD) and the Cartagena Protocol on Biosafety (CPB).